

So if I put a three there, families have three magnesium, you need to put a three there.
I have to there two there so that's four oxygen in total, which means they put to their to make four oxygen in total for this one magnesium and manganese are all by themselves, so they're going to be the easiest to balance, so I'm going to balance the oxygen's. So I have one carbon, one carbon that's balanced four hydrogen had to put it there to make four hydrogen, which means I now need to check my oxygen's. I tend to balance the carbon and hydrogen first oxygen last only because there's a single oxygen that's easy to balance.

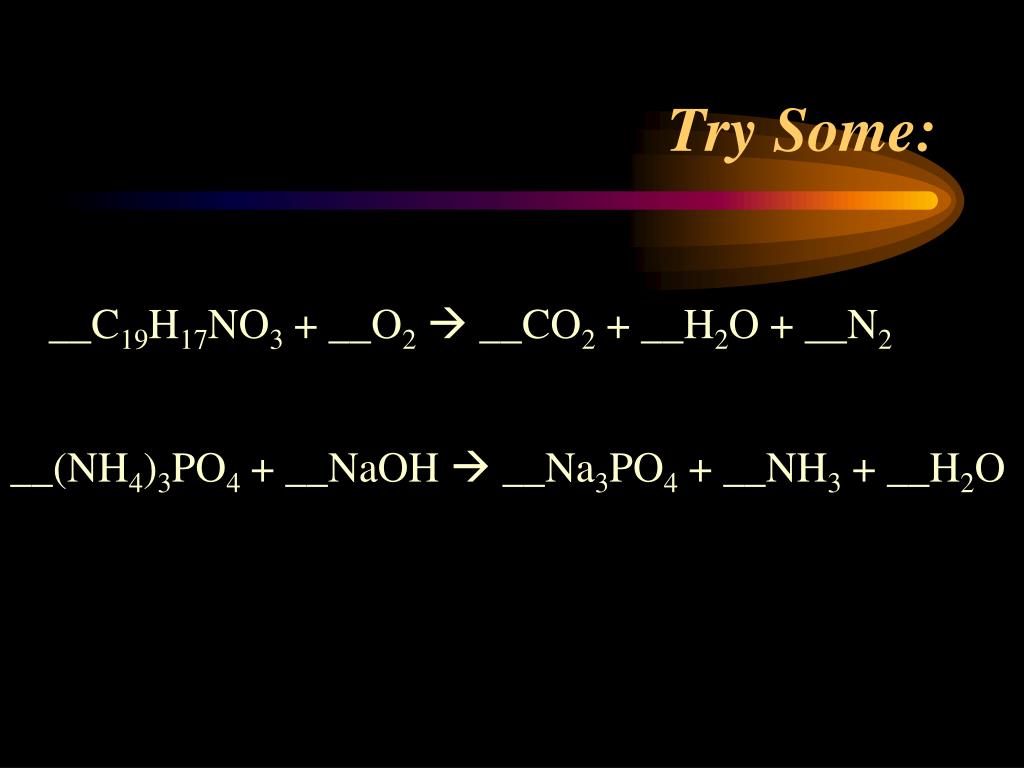
It's already balanced when I balance combustion reactions. Come on, silver, one, silver one nitrate, one nitrate, one, lithium one, lithium one hydroxide one hydroxide. So I put two there, that makes two iron Here, I have six chlorine and we had to put a three there to make six chlorine. I have the positive balance, that's two iron. So if I put a two there that makes two phosphate and I'm going to work it through. So I have to sodium to sodium to chlorine, to chlorine. So I had to put it to their to make to sodium. So there's one sodium Mon sodium one, sodium starts balanced one chlorine but I have to chlorine there so that means we're going to put a to their to make to chlorine but it throws off the sodium which I had balanced and now I have to sodium in one. And were the first one has no polly atomic ions. So I'm going to go through this systematically. We were asked to balance the following equation. The Algebraic Balancing Equations Method. the balanced equation for the reaction between hydragen and oxygen is: 2Hz 2h20 NOT 4Hz 202 4Hzo Eg: Wrte balanced chemical equation for the following reactions Ĥ months, 2 weeks ago Baloncing Chemical Equations Tips & Tricks Remember, when trying to balance coefficient equations, You can only change the value of the coefficient in front of the compound or lement, not - the subscripts Therefore, the balanced chemical equation becomes C3H8 + 5O2 3CO2 + 4H2O. When the equation balanced, the coefficients should be their lowest terms. Balance the element, other than hydrogen and oxygen that has the greatest number of atoms any reactant or product.īalance other elements,other than hydrogen and oxygen:īalance oxygen hydrogen, whichever one present in the combined state: Lrove until last whichever one present the uncombined state:Ĭheck that the equation balanced by counting the number of atoms of each element on each side of the equation SOLVED: Baloncing Chemical Equations Tips Tricks Remember, when trying to balance coefficient equations, You can only change the value of the coefficient in front of the compound or lement, not - the subscriptsīalance polyatomic ions as whole Eg: SOa should be balanced instead of balancing Sulfur and Oxygen separately.


 0 kommentar(er)
0 kommentar(er)
